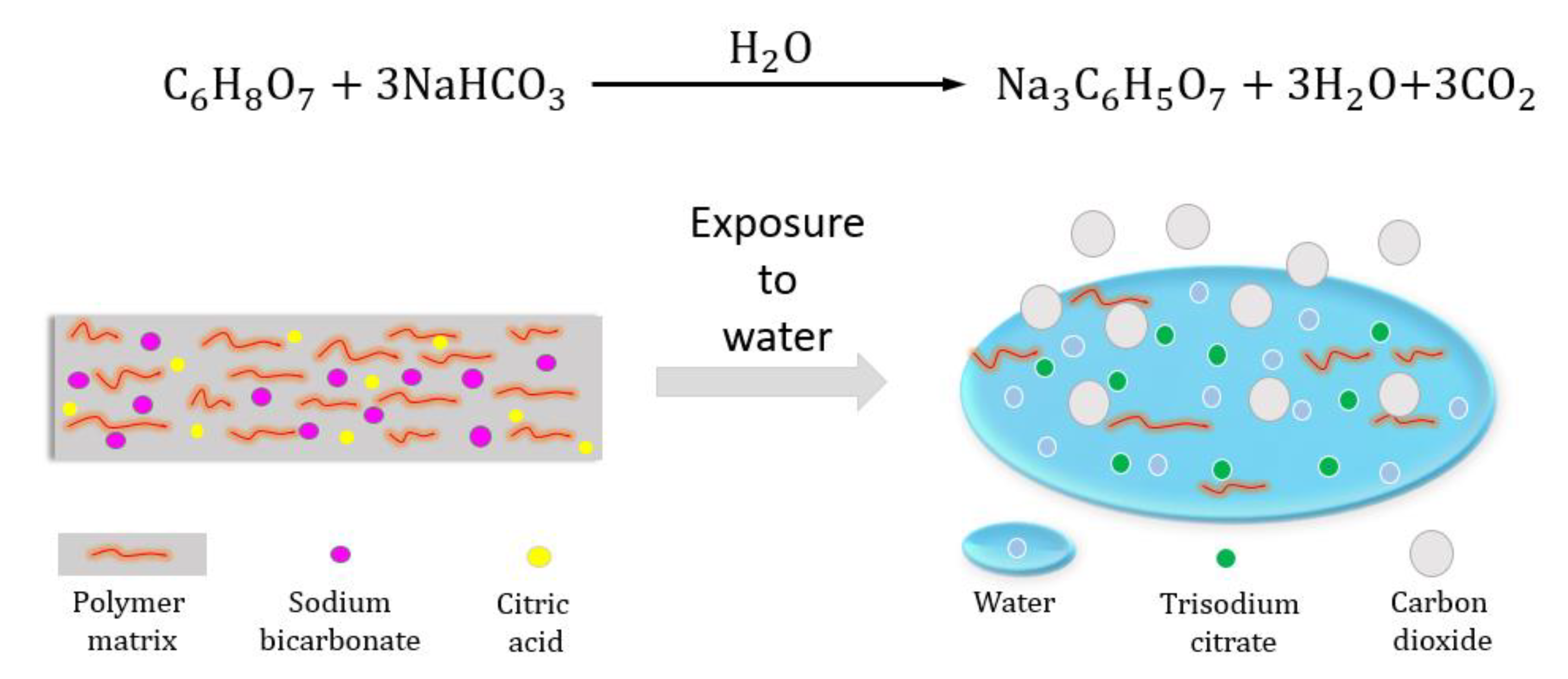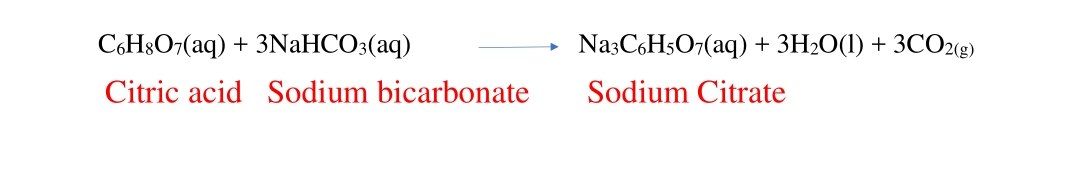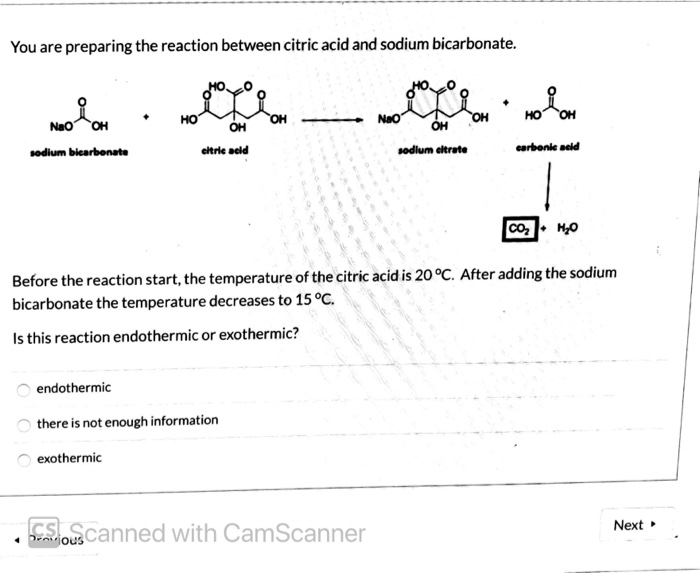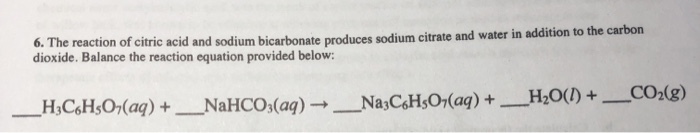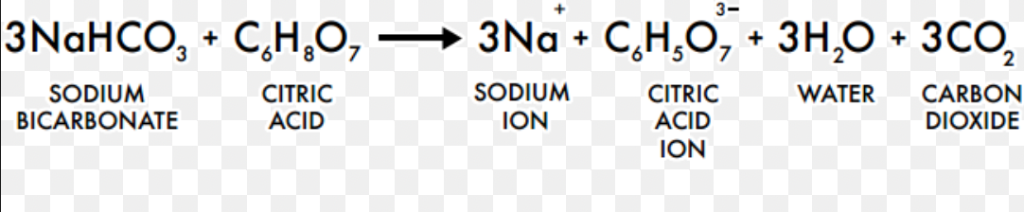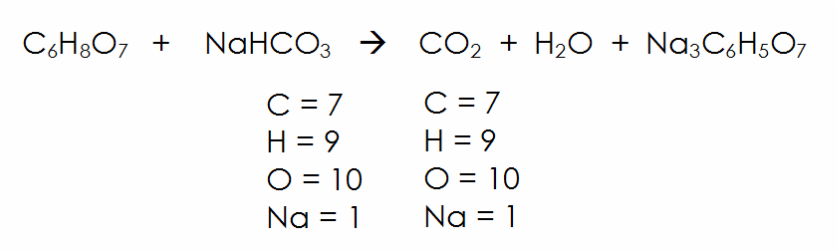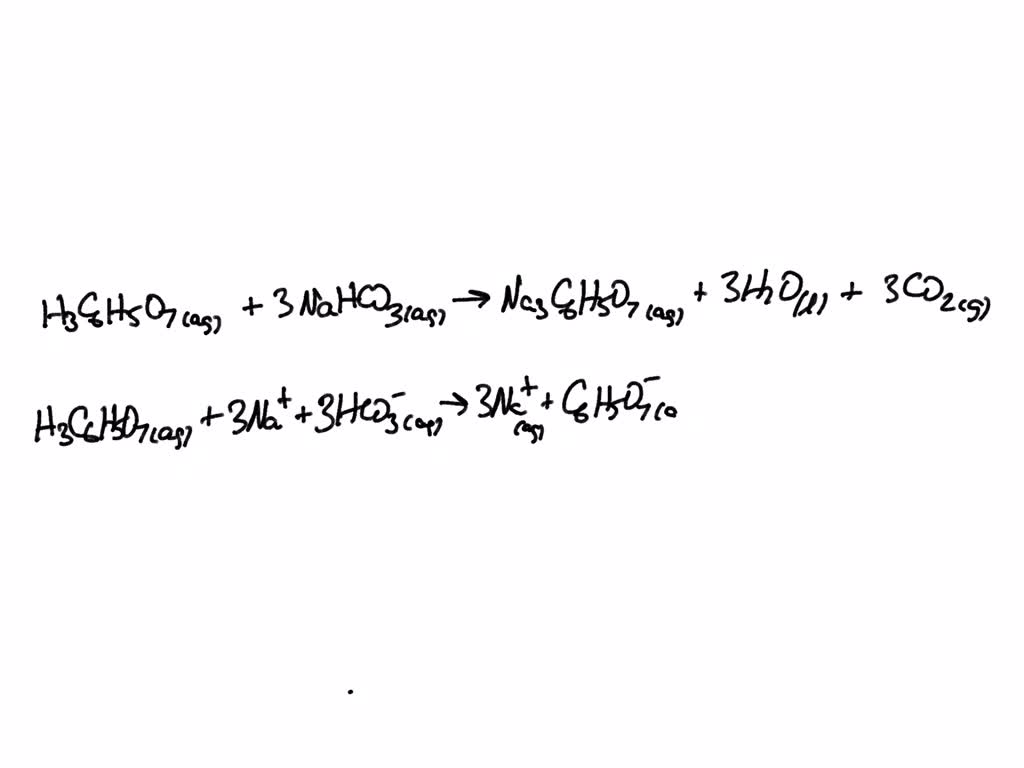
SOLVED: Write the balanced net ionic equation for the reaction between aqueous solutions of citric acid and sodium bicarbonate. H3C6H5O7 (aq) + NaHCO3 (aq) = Na3C6H5O7 (aq) + CO2 (g) + H2O (l)

Exogenous citrate can react with carbonic acid to form citric acid and... | Download Scientific Diagram

SOLVED: Alka Seltzer reaction involves citric acid and sodium bicarbonate (baking soda) according to the following unbalanced equation: HaCoHsOz NaHCOz > Na3CoHsOz HzO CO2 Starting from 1.3 g citric acid; how many

PhysiciansCare® 12-406 Alka-Seltzer® Anhydrous Citric Acid, Aspirin, Sodium Bicarbonate | Saf-T-Gard International, Inc.
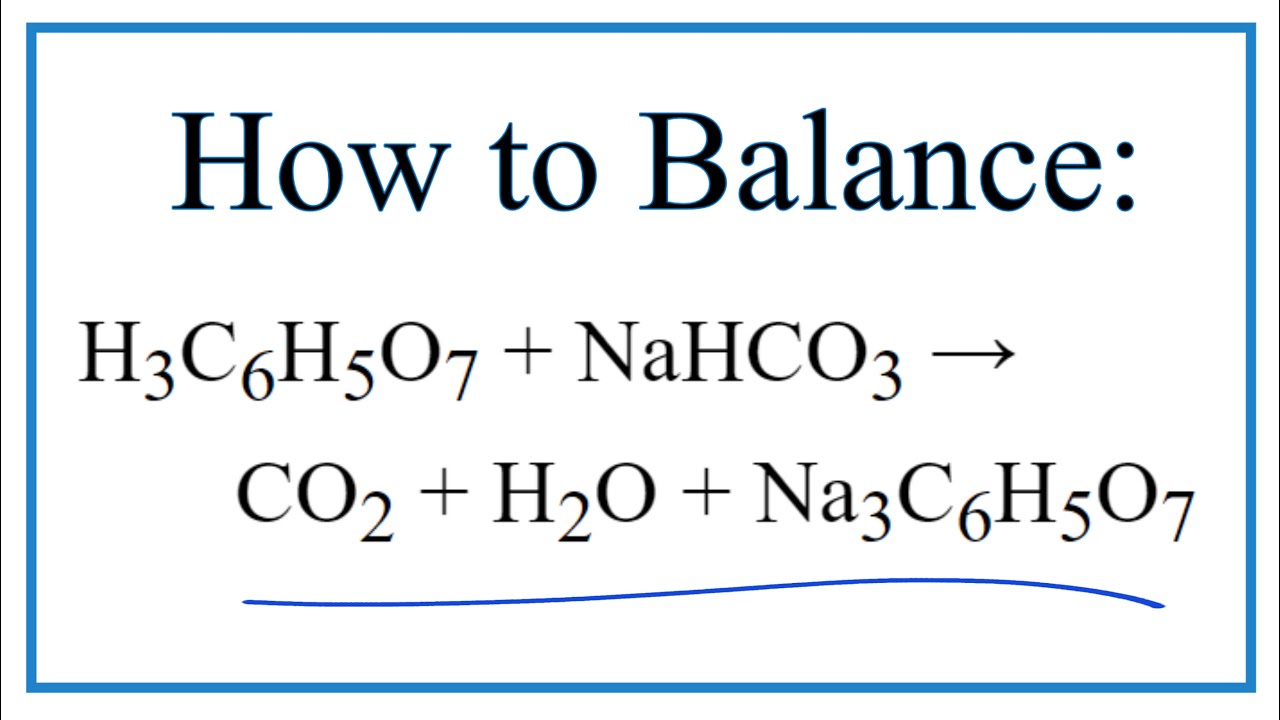
How to Balance H3C6H5O7 + NaHCO3 = CO2 + H2O + Na3C6H5O7 (Citric acid + Sodium bicarbonate ) - YouTube
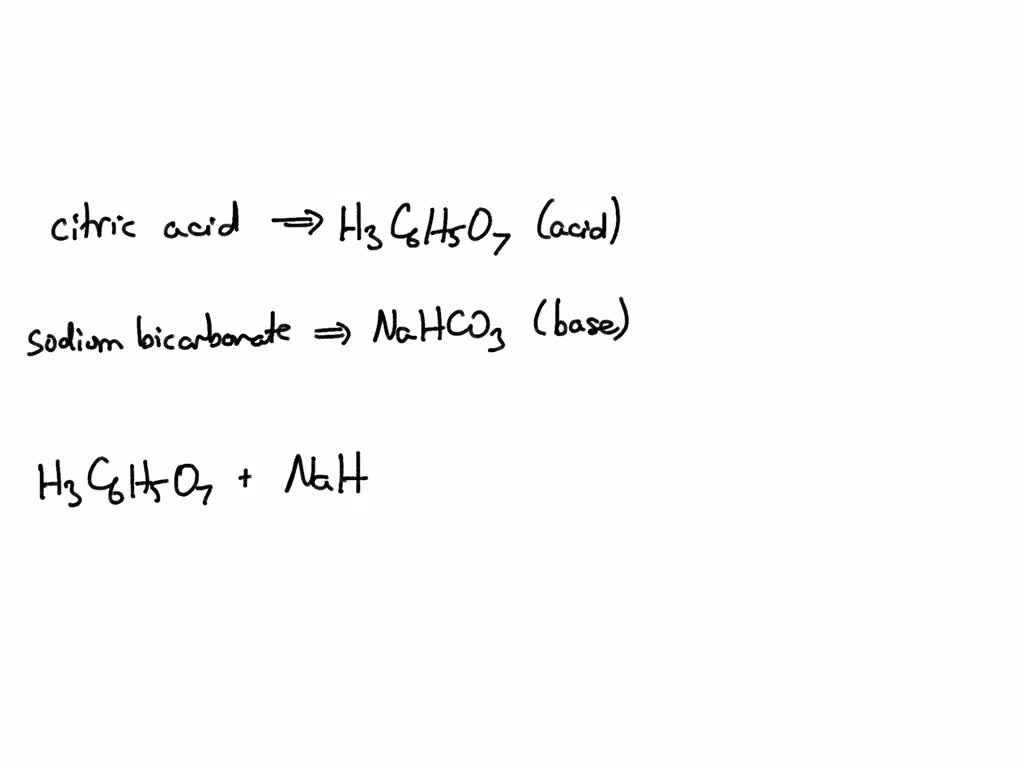
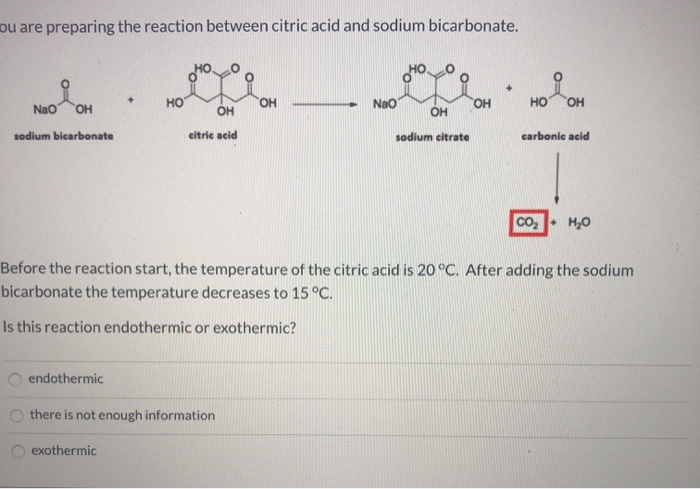
SOLVED: Write an equation for the neutralization of lemon juice (citric acid) with baking soda (sodium hydrogen carbonate or sodium bicarbonate). You equation should show a proton transfer to form carbonic acid

Dsc studies on the decomposition of chemical blowing agents based on citric acid and sodium bicarbonate - ScienceDirect